
Armenia: Only 16% of Health Supplements Sold via Social Media are State Registered
Biologically active supplements (BAS) and vitamins that are sold on social media networks in Armenia are not only unregistered by the state or registered in violation of the norms of technical regulations, but some are also considered drugs.
For three months, the Hetq Media Factory team researched biologically active supplements sold freely on social media in Armenia and found about 200 registered supplements, of which only 31 or 16% were licensed for sale.
At the same time, from June 2018-2021, Armenia's Food Safety Inspectorate (FSI) overseeing the BAS industry only found five unregistered supplements. One case was based on a Hetq publication.
Of 190 supplements, 31 are registered by the state
Biologically active supplements are considered food and are controlled by the Food Safety Inspectorate, regulated by the Technical Regulation of the Customs Union 021/2011 "On Food Safety".
In order to be sold in Armenia and in other EEU countries, they must be registered by the state, otherwise their sale is prohibited.
Products registered by the state have passed the conformity assessment stages, have been inspected, are safe, and have the right to appear on the market and be sold.
Unfortunately, in the Armenian market today, you can buy many supplements and vitamins that are not registered and are sold freely, especially through social media networks.
Vitamins, for example, depending on their dosage, can be either a biologically active supplement or a drug, in which case it must be prescribed by a specialist since an overdose of vitamins is dangerous and there is such a thing as a permissible daily dose.
In addition, drugs have a greater effect, chemical components, healing properties, and are controlled by the Ministry of Health.
BAS, on the other hand, have an herbal base, and the product is used as a supplement for preventative measures.
For March to May 2021, the Hetq Media Factory team researched 16 Facebook pages selling biologically active supplements.
- Vitamin House
- Vitamin.am
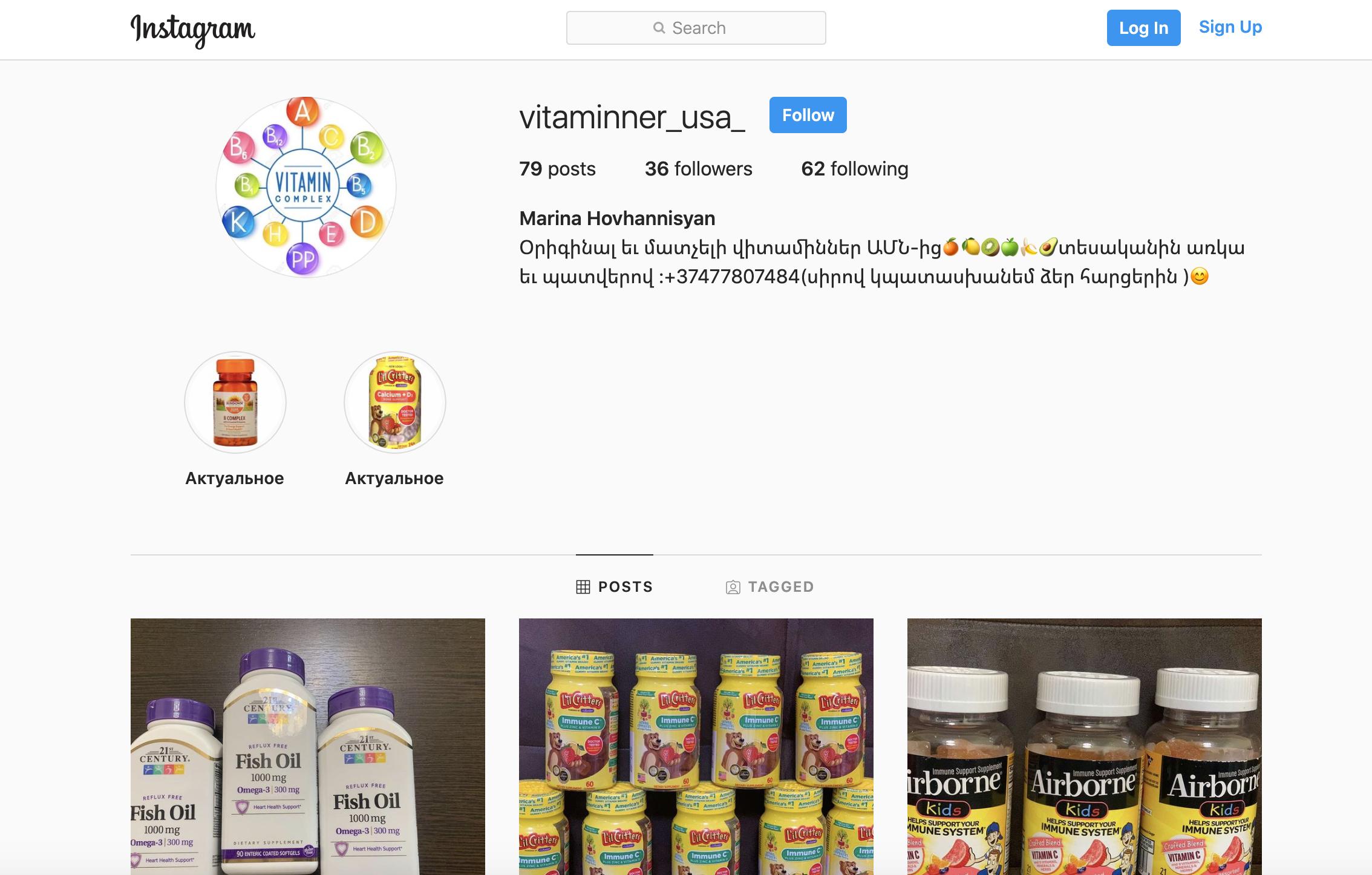
- Vitaminner.com
- Grocery Store Natural
- VITA Shop
- Vitamins and Supplements
- Healthcare, Vitamins for Life
- All in one
- Online doctor
- Vitaneed
- PH Luxe
- NEW VITA
- My vitamins
- All In One Online Shop
The two on instagram are:
Most of these pages were created in 2020 and this unregulated business was booming, since so many different medications and vitamins (for example, vitamin D, zinc) were recommended during the active period of the COVID-19 pandemic.
Business is still booming, as evidenced by the hundreds of comments, responses, and shares on those pages. Pages also often post advertisements.
Both supplements and specially formulated food considered sports food and medicine are sold through social media networks without a license.
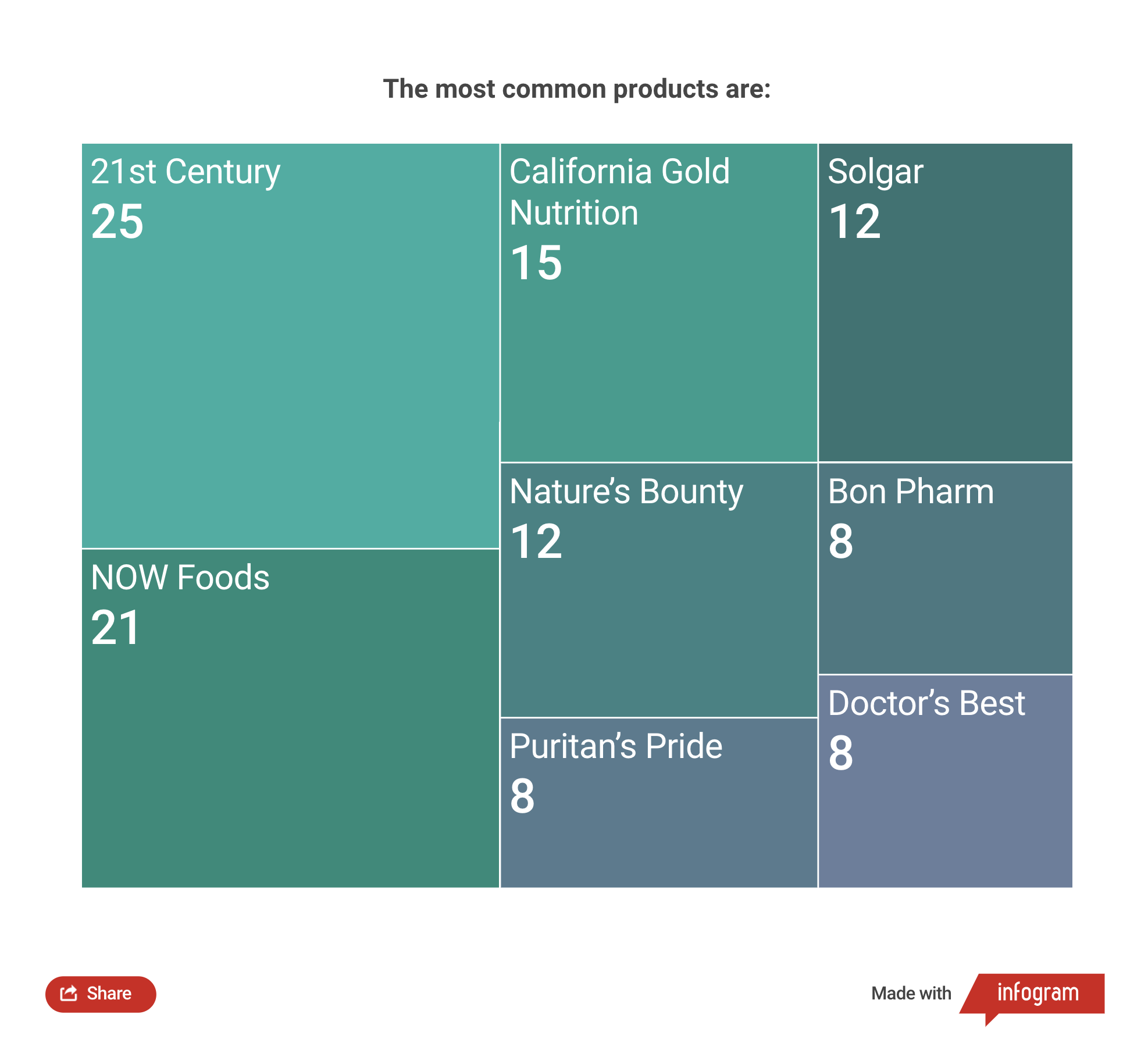
We investigated the 190 BAS that are on sale (not including duplicate products). Only 31 are registered by the state and have sale permits and 3 are registered but with violations.
Among them are two vitamins from Doctor's Best: one that comes with 188 capsules and the other with 360 capsules. Vitamin D 5,000 IU is registered as a supplement, though the dosage is that of a drug (see below for more information about these vitamins and dosages).
Another case involves the BAS Black Walnut Hulls, 500 mg, 100 veg that is manufactured by the Now company. A dosage of 605-616 mg is registered by the state, but the dose marked on the box is 500 mg, which is a violation. Whatever is marked on the box must correspond to what was indicated at the time of registration; otherwise, it is considered another product, since the package can’t be identified.
Our Hetq Media Factory team thus revealed 159 unregistered BAS in 3 months. It turns out that 84% of the supplements sold through social media network pages do not have mandatory state registration, making their sale prohibited. Only 16% are registered.
 |
 |
Unregistered Vitamin D in doses 2.5-10 times higher than allowed
As mentioned previously, the dosage of vitamins in particular is important because, depending on the dosage, they can also be drugs.
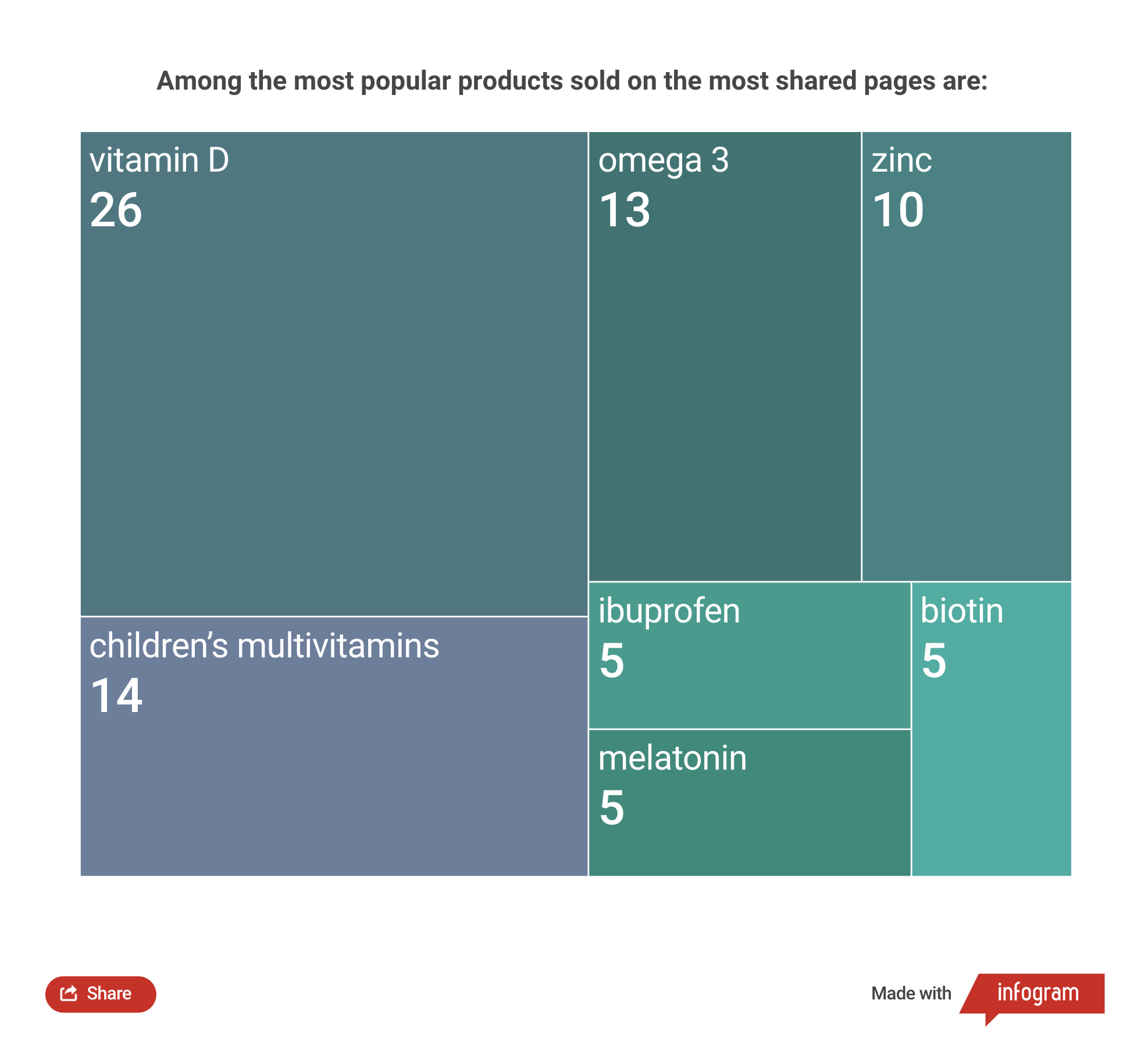
The sale of unregistered vitamin D3 is the most widespread and in demand. During our investigation, we identified 26 vitamin D products, of which 9 were found to be drugs owing to their high dosages.
Six have doses of 5,000 IU (international units).
- Doctor's Best brand, Vitamin D3 5,000 IU,125 mcg, 180 softgels, sold on the Vitamin House Facebook page;
- Doctor's Best brand, Vitamin D3 5,000 IU, 125 mcg, 360 softgels, sold on Vitaminner.com;
- Now Company, Vitamin D-3 5,000 IU, 120 softgels, sold by Vitamin House;
- California Gold Nutrition brand, Vitamin D3 125 mcg (5,000 IU), 360 Fish gelatin softgels, sold on Vitaminner.com;
- California Gold Nutrition brand, Vitamin D3 125 mcg (5,000 IU), 90 fish gelatin softgels, sold on Vitaminner.com;
- Solgar brand, Vitamin D3 5,000 IU, 100 softgels, sold on Vitaminner.com.
Moreover, vitamin D3 is also sold on social media networks, one with a dosage of 10,000 IU (Now brand, sold on the Vitamin.am page) and two with a dosage of 50,000 IU (Forest Leaf brand, sold on the PH Luxe page, and the Super Nutrition company brand, sold on Vitaminner.com). They have high therapeutic doses and are not recommended without a doctor's prescription.
It is also worth mentioning that the prices of goods sold on social media pages differ from that of pharmacies. For example, 180 capsules of American Doctor's Best (5,000 IU) costs 27,000 drams at Alfa Pharm. On social media, they are 7,000 drams–4 times as cheap–and on online platforms abroad they are twice as cheap as that: The same 180 capsules of Doctor’s Best (5,000 IU) costs $6-6.50 or 2,900-3,200 drams.
We decided to get in touch with those selling BAS on social media, posing as an ordinary buyer.
The Vitamins for Life page has 1,871 followers, and in the description section they write, “only original vitamins and nutritional supplements from the USA”. In a conversation with us, the seller introduced himself as the importer, saying that he himself orders everything from USA-based manufacturers’ websites, so “there are no false claims”.
We were interested in the three different brands of Vitamin D on their page: Doctor's Best, Vitamin D3, 5,000 and 2,000 IU; California Gold Nutrition, Vitamin D3, 5,000 IU; and Now Foods, Vitamin D3, 5,000 IU.
Only the first one mentioned (Doctor's Best, Vitamin D3, 5,000) has a registered trademark, but even that is in violation, because according to the Technical Regulation of the Customs Union 021/2011, the vitamin dosage should not exceed “50% of the therapeutic dose”, which it does.
In the case of vitamin D, 2,000 IU is considered the therapeutic dose in various medical directories and publications. That is, 5,000 IU of vitamin D can’t be considered a preventative amount; it is not a supplement but rather, a drug. This was confirmed by the Emil Gabrielyan Expert Center for Drugs and Medical Technologies. They stated that the requirements as per the Technical Regulation of the Customs Union 021/2011 has applied to all registered vitamin D products since December 2019, which allows for BAS to have a dosage up to 2,000 IU.
According to the seller/importer, the vitamin Doctor's Best, Vitamin D3, 5,000 is consumed the most in Armenia. We were told that all the products are sold at pharmacies for 23,000-28,000 drams. “Natalie Pharm, Alfa Pharm, Vaga-Pharm, Levon Lamara–I’ve seen them in all those [pharmacies],” said the seller/importer.
When we asked about the differences between vitamin D dosages 2,000 IU and 5,000 IU, he inquired about why we wanted to take the 5,000 dose and asked whether we had been tested or wanted it for preventative purposes.
We answered that we wanted it for preventative purposes, since that’s the function of BAS, to which he responded with a recommendation to take the 5,000 IU dosage if we had never taken vitamin D before.
We mentioned having a stomach problem without clarifying the problem and asked if it would be harmful to take the vitamin in this case. The seller said that it has no contraindications and will help with everything. “I have even read that it is good for the stomach as well. Practically everyone these days lacks [vitamin D],” he said.
We inquired as to whether the products being sold were drugs or biological supplements, and it was clear that the seller himself didn’t know the answer, since he called them “hormones” and noted that people also say “supplements”.
When we said that 5,000 IU is already considered a drug, he objected and said, “No, it’s not a drug”.
We asked him to send us a product certificate, to which the seller provided the Doctor’s Best official website and, instead of sending the California Gold Nutrition certificate, he referred us to the Amazon product page.
- I looked up this drug on the EEU State Register of State Registration and didn’t see it. Can you help me find it?
- It’s American. Maybe that’s why it’s not there. Which one did you look at: California Gold or D3?
- California Gold. Practically none of those products are registered. Doctor’s Best is also American, but the 5,000 is registered, while the other one isn’t.
- I don’t know. It’s one of those brands. The 2,000 isn’t registered?
- No
- And who is the one registering it? The importer?
- Yes, you’re also the importer, right? In Armenia, over 2,000 is considered a drug, which requires a license to sell. You need a license to sell BAS as well. Will you send over that license?
- We’re not able to get licensing. That costs 2,000,000 drams. We only bring over a few [packets].
- You’re only allowed to bring it for personal use, not to sell.
- The importing company that gets a license–they make the price 25,000-29,000 drams.
We repeated that in Armenia a vitamin D product over 2,000 IU is considered a drug. The seller said that he didn’t know that and could not find any information about that. Then he explained that he came from Stepanakert “after the war” and started selling BAS. However, it should be noted that the Vitamins for Life page on Facebook was created on August 29, 2020, and the first batch of products was put up for sale on September 18, 2020, before the war started.
The next seller who we talked to was through the VITA Shop Vitamins and Supplements Facebook page, which was created on March 3, 2019 and has 3,330 followers.
The Vitamin D products sold here were a little different: Dosages were 5-10 times higher than what is allowed for supplements.
They had the same Doctor's Best, Vitamin D3, 5,000 IU and California Gold Nutrition, Vitamin D3, 5,000, as well as Solgar Vitamin D3 10000 and Now Foods brands with the same dosages.
We asked, “What high doses do you have?” They answered that they also have vitamin D 50,000 IU but didn’t specify the brand.
At first, the conversation proceeded like the other one we had․ We were assured that it would not be harmful for digestive problems. The seller said, “There are no contraindications as such.” However, he had a different suggestion for how to take the medication: to drink it with food, since “it is a dietary supplement”.
However, when we said that the dose of vitamin D was above 2,000 IU and it is considered a drug in Armenia, not a biological supplement, it turned out that the seller was not aware of the established requirements and norms.
Here are some of their answers:
— Who told you that?
— How do you know that 2,000 IU is considered a drug? Have you read it somewhere or is it something you were told?
— What standards are you referring to? There are different criteria, but that has nothing to do with what you said.
When we sent the documents that show proof of the given definitions, requirements, and norms, he took on an even more aggressive tone.
He asked us, “Why are you searching for all that on the Internet? Why not go to the store and buy it?” He also said other things like: “It’s like you’re looking for a certain answer.”
Our dialogue came to a dead end, because the person speaking on behalf of the page replied that what they sell doesn’t concern us, that it doesn’t matter if they have the appropriate license or not, and that they hadn’t violated any laws because they hadn’t sold us anything.
In addition to vitamins, they also sell the non-steroidal anti-inflammatory drug known as ibuprofen over social media.
We found five such products as a result of our investigation:
- Advil Liqui-gels, Solubilized Ibuprofen Capsules 200 mg, 120 capsules, sold on the Vitaminner.com Facebook page;
- Advil Liqui Gels, Solubized Ibuprofen Capsules, 200 mg, 160 liquid filled capsules, sold on the VITA Shop Facebook page;
- Equate Ibuprofen 200 mg, 200 coated tablets, sold on the Healthcare Facebook page;
- Advil Ibuprofen tablets, sold on the Vitaneed Facebook page;
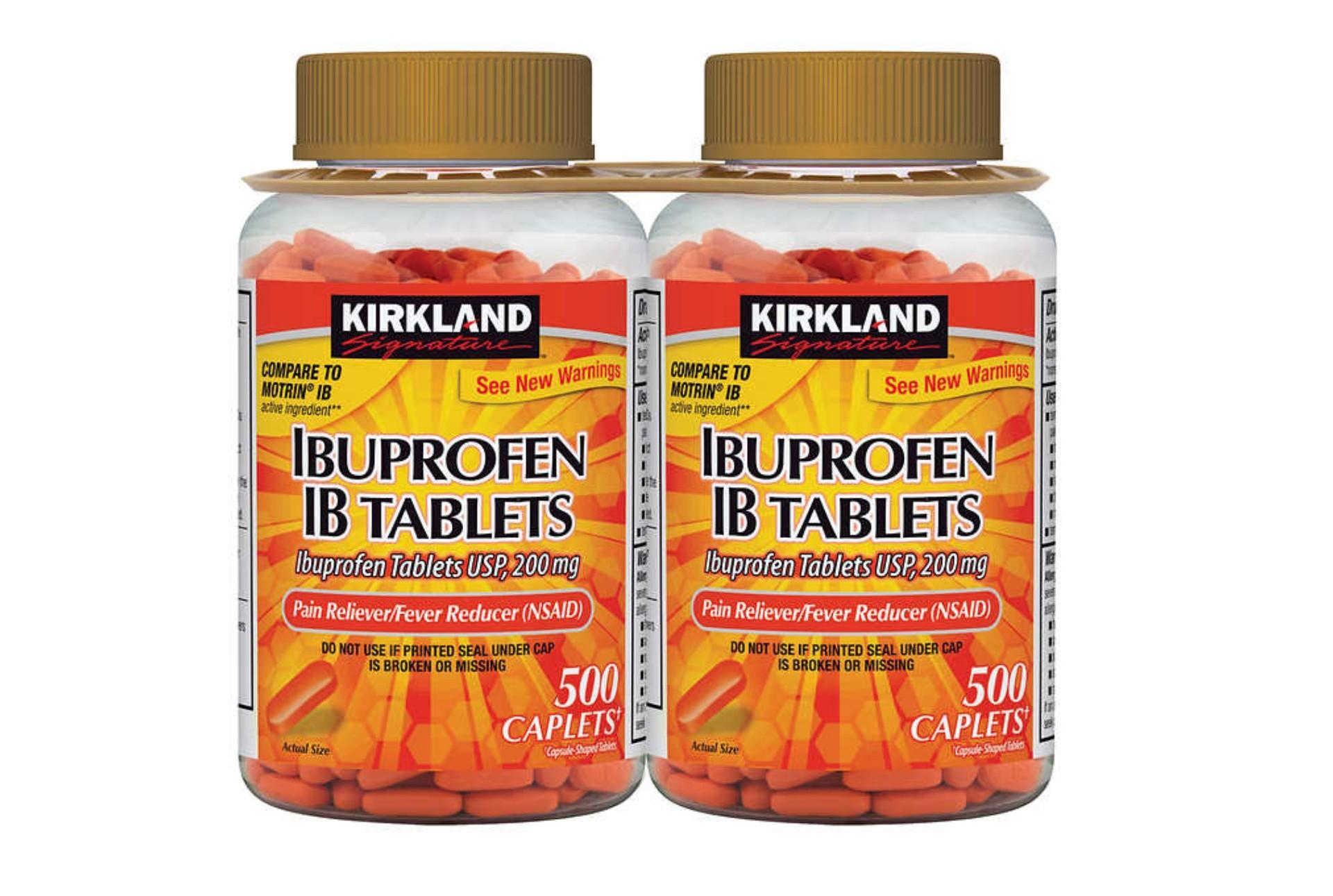
- KIRKLAND Signature IBUPROFEN IB TABLETS, sold on the PH Luxe Facebook page.
The Food Safety Inspectorate has uncovered 5 unregistered supplements in 3 years
In parallel with our 3-month study, we sent a request to the Food Safety Inspectorate (FSI), which supervises the sector, asking them to inform us about violations they have recorded in the BAS industry between 2018-2021.
In response to our inquiry, the FSI informed us that from June 15, 2018 to June 15, 2021 they uncovered 5 unregistered supplements that “were not subject to conformity assessments”.
More specifically, the FSI uncovered the BAS Penoxal Forte and Pen BG in 2020 and the above-mentioned Doctor’s Best brands Vitamin D3 5,000 IU and Vitamin D3 1,000 IU in 2021.
One BAS is the unregistered Mycelix that the FSI revealed after the Hetq publication. The materials were transferred over to the police and a criminal case was initiated.
The FSI mainly applies administrative measures such as fines and warnings when there’s an issue of noncompliance.
We also asked the FSI about measures they take to clamp down on the sale of various BAS that are not registered by the state but actively sold over social media and how they carry out safety monitoring.
We were informed that, according to the Law on Trade and Services, individuals can engage in e-commerce only if they are registered as an entrepreneur and have electronic payment documents. The FSI has repeatedly appealed to sellers on social media, but they have refused to provide any information about their workplace or business activities.
“Measures have been taken by the FSI to uncover the legal status of online businesses and their place of operation. However, it has not been possible, because those selling biologically active supplements via e-commerce platforms do not provide any information about their activities.”
The information collected by the FSI about BAS sold on electronic platforms was provided to the police in 2020.
Authors: Christian Ginosyan, Lilit Sareents Aleksanyan, Mary Andriasyan
Coordinator: Tatev Khachatryan
 Videos
Videos Photos
Photos



Comments (1)
Write a comment